Well to be more specific, female on antidepressant medication. Sexual dysfunction is a common antidepressant adverse effect, occur about 30-70% of major depression patients. This leads to non-compliance problem.
Antidepressant medication needs a long period of time before it could exert its full effect. Imagine this scenario. A major depression patient just started to take the antidepressant medication. At the initial stage (1-2 weeks), she still feeling depressed as the full effect of the medication is delayed. However at the same time, she develops sexual dysfunction such as orgasm delay, decreased libido (sexual drive), difficulty with arousal and anorgasmia (no orgasm) because of that medication that she took. As a result, she feels depress even more. And being a typical patient, she stops or skips in taking the antidepressant because of that sexual dysfunction effect. This will further delay the already long period to achieve the peak effect of antidepressant medication. This will result in much longer time frame to treat her major depression.
But dont worry, theres hope. In recent case reports and small studies, its shows that women taking Sildenafil (Viagra) group appeared to suffer sexual dysfunction less than the placebo group! Well, a further large, randomise control studies need to be done before this indication could be approved. But it will definitely help to bring down the anti-depressant non-compliance problems.
Whatever it is, the idea of the little blue pills for females just tickles me. haha.
The Study Highlight
- Inclusion criteria were ages 18 to 50 years, major depressive disorder in remission, taking SRIs for at least 8 weeks, persistent sexual dysfunction for at least 4 weeks, and regular sexual activity (at least twice a month).
- Exclusion criteria were other sexual disorder, sexual dysfunction before use of SRIs, genital anatomic abnormality, hysterectomy with or without ovariectomy, substance abuse, major relationship change, partner with sexual dysfunction, use of hormonal therapy, and psychiatric and serious medical disorders.
- Major depressive disorder in remission and sexual dysfunction (impaired desire, arousal, and orgasm and sexual pain) were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria.
- Women were recruited via outpatient and media advertising.
- Women received a physical examination including the Papanicolaou test and blood tests at baseline.
- The primary outcome was difference in score for the Clinical Global Impression scale adapted for sexual function.
- Secondary outcomes were differences in scores for the Sexual Function Questionnaire, the Arizona Sexual Experience scale-female version, and the University of New Mexico Sexual Function Inventory-female version.
- The 17-item Hamilton Depression Rating scale was administered at baseline and at weeks 2, 4, and 8 to monitor depression.
- Patient-recorded event logs were reviewed for frequency and percentage of successful intercourse attempts and number of satisfactory attempts at orgasm.
- 98 women met inclusion criteria and were randomly assigned to sildenafil (n = 49) or placebo (n = 49).
- Women were instructed to take 1 to 2 tablets 1 to 2 hours before anticipated intercourse (not more than once daily).
- Mean age was 36.7 years, and they had been taking antidepressants for a mean of 27.7 months.
- 83% to 93% of women had a partner, fewer than 20% smoked, more than 80% drank alcohol, and 78% to 90% were premenopausal.
- 77.6% completed the study (75.5% in the placebo group and 79.6% in the sildenafil group).
- The prevalence of sexual problems was high at baseline: mean number of problems was 3.0 for the sildenafil group and 2.8 for the placebo group, and 95.8% of women complained of more than 1 problem.
- The difference from baseline to endpoint in the mean change in Clinical Global Impression scale improvement in sexual function was 0.8 (P = .001), with a difference of 1.91 for the sildenafil group vs 1.10 for the placebo group.
- Women in the sildenafil group had a higher mean improvement in sexual function vs women on placebo for all domains except pain on the Sexual Function Questionnaire.
- In the Arizona and University of New Mexico questionnaires, the ability to reach orgasm and experience orgasm satisfaction was significantly better for those in the sildenafil group.
- The mean differences from baseline between the 2 groups were 0.5 (P = .01) for the Arizona questionnaire and 0.7 (P = .01) for the New Mexico questionnaire.
- At baseline, the 2 groups had similar Hamilton depression scores.
- Scores remained similar at the end of the study.
- No recurrence or relapse of major depression occurred in any of the women.
- Women whose sexual function improved had a higher mean baseline level of testosterone.
The most common adverse event was headache reported by 43% in the sildenafil group and 27% in the placebo group (P = .09), followed by flushing (24% vs 0%; P < .001), dyspepsia (12% vs 0%; P = .01), nasal congestion (37% vs 6%; P < .001), and transient visual disturbance (14% vs 2%; P = .03). - No serious adverse effects were reported.
Source: Medscape Pharmacist




 Next, you need to cut them accordingly because there is a safety bar inside your inner panel that prevent u from sticking the whole piece. The dimension is mentioned in the following pictures. [read: this is only true for Proton Satria model. Other cars, you need to inspect for yourself and determine the dimension]
Next, you need to cut them accordingly because there is a safety bar inside your inner panel that prevent u from sticking the whole piece. The dimension is mentioned in the following pictures. [read: this is only true for Proton Satria model. Other cars, you need to inspect for yourself and determine the dimension]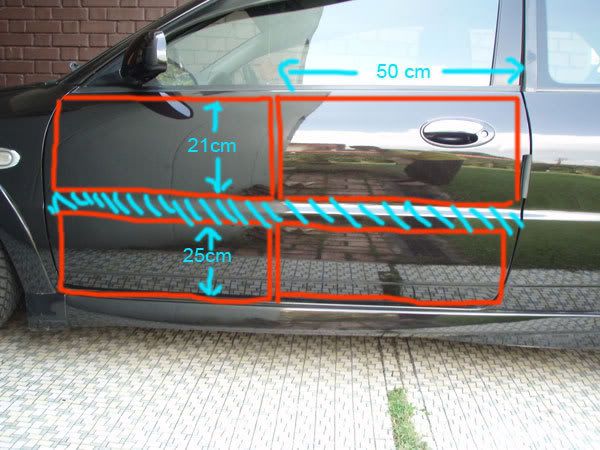
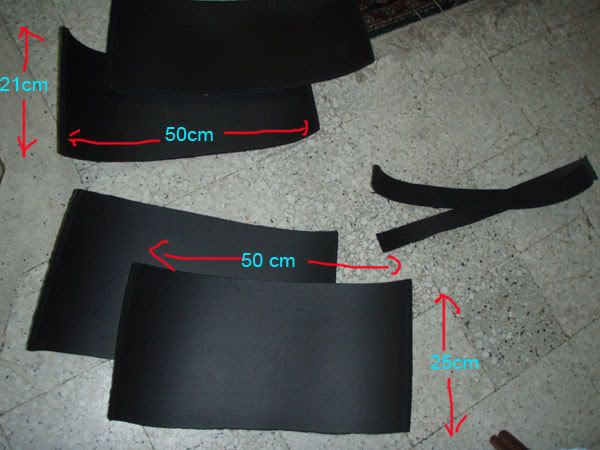
 Next, peel the adhesive part to reveal the sticky part
Next, peel the adhesive part to reveal the sticky part then stick it according to the 3rd picture and walla, you are done with the panels.
then stick it according to the 3rd picture and walla, you are done with the panels. Next part, soundproofing ur engine bay. U need a ready made/cut soundproofing material. Bought mine for RM38 at Brothers. If u want to save even more, u can buy another 2 50x50cm materials instead and cut it yourself. You need to trace it first to make a template. This is too time consuming and hassle for me and not worth my RM13.
Next part, soundproofing ur engine bay. U need a ready made/cut soundproofing material. Bought mine for RM38 at Brothers. If u want to save even more, u can buy another 2 50x50cm materials instead and cut it yourself. You need to trace it first to make a template. This is too time consuming and hassle for me and not worth my RM13. Next is to figure out which match with which section. Its like playing jigsaw puzzle! When u have idenfify them, peel the adhesive and stick it! its really FUN!
Next is to figure out which match with which section. Its like playing jigsaw puzzle! When u have idenfify them, peel the adhesive and stick it! its really FUN!
 and WALLA, you are done! Your car will feel more comfortable to your ears and feel more luxurious. Just dont expect a total silence as sound will still enter the car via other body part and windows, and also do remember, those luxurious car uses very expensive tyres, which is very quiet as compared to our normal cheap tyres. (like my Silverstone tyres, its the cheapest around, but the loudest too)
and WALLA, you are done! Your car will feel more comfortable to your ears and feel more luxurious. Just dont expect a total silence as sound will still enter the car via other body part and windows, and also do remember, those luxurious car uses very expensive tyres, which is very quiet as compared to our normal cheap tyres. (like my Silverstone tyres, its the cheapest around, but the loudest too)



 After the undercoat layer is well dried, apply your color of choice. Again, Ive chosen flat black. As explained in previous D.I.Y post, apply multiple thin layer of paint with 10-15 minutes of interval for drying.
After the undercoat layer is well dried, apply your color of choice. Again, Ive chosen flat black. As explained in previous D.I.Y post, apply multiple thin layer of paint with 10-15 minutes of interval for drying.


 a motorcycle clipped my cannard. haih.
a motorcycle clipped my cannard. haih. I guess I parked toooo close with the roadside curb
I guess I parked toooo close with the roadside curb
 Start spraying. I used the same color as the undercoat because I'm too lazy+stingy to buy another spray can. Spray it layer by layer, preferably multiple thin layer seperated by about 5-10 minutes of drying. This will produce better result as compared to 1 thick layer of paint.
Start spraying. I used the same color as the undercoat because I'm too lazy+stingy to buy another spray can. Spray it layer by layer, preferably multiple thin layer seperated by about 5-10 minutes of drying. This will produce better result as compared to 1 thick layer of paint.






 and the results.. Zero swirl marks!
and the results.. Zero swirl marks!


